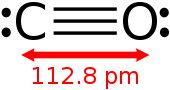| Carbon monoxide |
|---|
| |
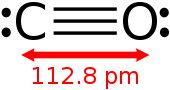 |
|
| Other names | Carbon monooxide
Carbonous oxide
Carbon(II) oxide
Carbonyl
Flue gas
Monoxide |
| Identifiers |
|---|
| CAS number | 630-08-0 |
| PubChem | 281 |
| EC number | 211-128-3 |
| KEGG | D09706 |
| MeSH | Carbon+monoxide |
| ChEBI | CHEBI:17245 |
| RTECS number | FG3500000 |
| SMILES | [C-]#[O+] |
|
| Beilstein Reference | 3587264 |
| Gmelin Reference | 421 |
| Properties |
|---|
| Molecular formula | CO |
| Molar mass | 28.010 g/mol |
| Appearance | colorless gas |
| Odor | odorless |
| Density | 789 kg/m3, liquid
1.250 kg/m3 at 0 °C, 1 atm
1.145 kg/m3 at 25 °C, 1 atm |
| Melting point | −205.02 °C, 68 K, -337 °F |
| Boiling point | |
| Solubility in water | 27.6 mg/L (25 °C) |
| Solubility | soluble in chloroform, acetic acid, ethyl acetate, ethanol, ammonium hydroxide, benzene |
| kH | 1.04 atm·m3/mol |
| −9.8·10−6 cm3/mol |
| Refractive index (nD) | 1.0003364 |
| Dipole moment | 0.122 D |
| Thermochemistry |
|---|
Std enthalpy of
formation ΔfHo298 | −110.5 kJ/mol |
Std enthalpy of
combustion ΔcHo298 | −283.4 kJ/mol |
Standard molar
entropy So298 | 197.7 J/(mol·K) |
| Specific heat capacity, C | 29.1 J/(K·mol) |
| Hazards |
|---|
| EU classification |  F+ F+  T+ T+ |
| NFPA 704 | |
| R-phrases | R61 R12 R26 R48/23 |
| S-phrases | S53 S45 |
| Explosive limits | 12.5–74.2% |
U.S. Permissible
exposure limit (PEL) | TWA 50 ppm (55 mg/m3) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |