• phosphate rock
• phosphate rocks
• phosphate used
• phosphate image
• phosphate in florida
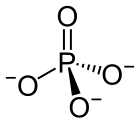
• phosphate chemical
• phosphate uses
• phosphate natural resources
• phosphate mine
• phosphate means
Not Finding Your Answer?
Post It On KidzTalk Homework Help
Post It On KidzTalk Homework Help
Report a search problem
mobile version
Copyright 2005-2024 KidzSearch.com