
|
 | ||||||||||||||||
| General properties | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈhiːliəm/ | |||||||||||||||
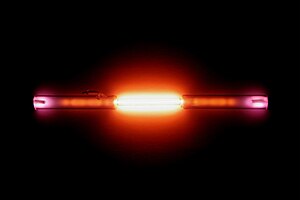
| Appearance | colorless gas, exhibiting a red-orange glow when placed in an electric field | |||||||||||||||
| Standard atomic weight (Ar, standard) | 4.002602(2) | |||||||||||||||
| Helium in the periodic table | ||||||||||||||||
| ||||||||||||||||
| Atomic number (Z) | 2 | |||||||||||||||
| Group | group 18 (noble gases) | |||||||||||||||
| Period | period 1 | |||||||||||||||
| Block | s-block | |||||||||||||||
| Element category | noble gas | |||||||||||||||
| Electron configuration | 1s2 | |||||||||||||||
Electrons per shell | 2 | |||||||||||||||
| Physical properties | ||||||||||||||||
| Phase at STP | He: Gas | |||||||||||||||
| Melting point | 0.95 K (−272.20 °C, −457.96 °F) (at 2.5 MPa) | |||||||||||||||
| Boiling point | 4.222 K (−268.928 °C, −452.070 °F) | |||||||||||||||
| Density (at STP) | 0.1786 g/L | |||||||||||||||
| when liquid (at m.p.) | 0.145 g/cm3 | |||||||||||||||
| when liquid (at b.p.) | 0.125 g/cm3 | |||||||||||||||
| Triple point | 2.177 K, 5.043 kPa | |||||||||||||||
| Critical point | 5.1953 K, 0.22746 MPa | |||||||||||||||
| Heat of fusion | 0.0138 kJ/mol | |||||||||||||||
| Heat of vaporization | 0.0829 kJ/mol | |||||||||||||||
| Molar heat capacity | 20.78 J/(mol·K) | |||||||||||||||
Vapor pressure (defined by ITS-90)
| ||||||||||||||||
| Atomic properties | ||||||||||||||||
| Oxidation states | 0 | |||||||||||||||
| Electronegativity | Pauling scale: no data | |||||||||||||||
| Ionization energies |
| |||||||||||||||
| Covalent radius | 28 pm | |||||||||||||||
| Van der Waals radius | 140 pm | |||||||||||||||
| Spectral lines of helium | ||||||||||||||||
| Other properties | ||||||||||||||||
| Natural occurrence | He: Primordial | |||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) | |||||||||||||||
| Speed of sound | 972 m/s | |||||||||||||||
| Thermal conductivity | 0.1513 W/(m·K) | |||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||
| Magnetic susceptibility | −1.88·10−6 cm3/mol (298 K) | |||||||||||||||
| CAS Number | 7440-59-7 | |||||||||||||||
| History | ||||||||||||||||
| Naming | after Helios, Greek Titan of the Sun | |||||||||||||||
| Discovery | Pierre Janssen, Norman Lockyer (1868) | |||||||||||||||
| First isolation | William Ramsay, Per Teodor Cleve, Abraham Langlet (1895) | |||||||||||||||
| Main isotopes of helium | ||||||||||||||||
| ||||||||||||||||
Helium is a chemical element. It has the chemical symbol He, atomic number 2, and atomic weight of about 4.002602. There are 9 isotopes of helium, only two of which are stable. These are 3He and 4He. 4He is by far the most common isotope.